Thin Layer Chromatography
Thin‑layer chromatography
involves the same principles as column chromatography, it also is a form of
solid‑liquid adsorption chromatography. In this case, however, the solid
adsorbent is spread as a thin layer (approximately 250 um) on a plate of glass
or rigid plastic. A drop of the solution to be separated is placed near one
edge of the plate, and the plate is placed in a container, called a developing
chamber, with enough of the eluting solvent to come to a level just below
the "spot." The solvent migrates up the plate, carrying with it
the components of the mixture at different rates. The result may then be a
series of spots on the plate, falling on a line perpendicular to the solvent
level in the container (see, for example, the following Figure). The retention
factor (Rf) of a component can then be measured as indicated in the figure
below.
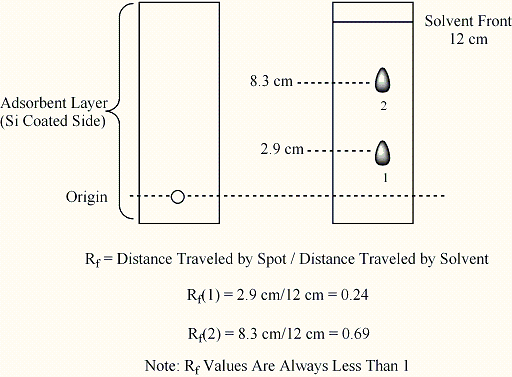
This chromatographic technique
is very easy and rapid to perform. It lends itself well to tine analysis of
mixture composition and may also be used to advantage in determining the best
eluting solvent for subsequent column chromatography.
However, it should be borne in mind that volatile compounds (bp <
100EC) cannot be analyzed by TLC.
The same solid adsorbents
used for column chromatography may be employed for TLC; silica gel and alumina
are the most widely used. The adsorbent is usually mixed with a small amount
of "binder"--for example, plaster of Paris, calcium sulfate, or
starch--to ensure adherence of the adsorbent to the plate. The plates may
be prepared before use, or commercially available prelayered plastic sheets
may be used. In either case, the plates or sheets should be dried for an hour
or more in an oven at 110EC before use. This procedure removes moisture that is adsorbed on the adsorbent
and produces a surface that is more effective in adsorbing and separating
the components of the mixture being analyzed. It should be remembered that
the eluting power required of the solvent is directly related to the strength
of adsorption of the components of the mixture on adsorbent.
A distinct advantage of
TLC is the very small quantity of sample required. A lower limit of detection
of 10‑9 g is possible in some cases. The spot of sample must
be applied to the plate with care; it should not be large, for the same reason
that the amount of sample placed at the top of a column should not be too
large. However, larger samples such as 0.5 mg may
be used on larger TLC plates, which have thicker coats of adsorbent. In these cases isolation of the components may be achieved
by scraping the separated spots from the plate and eluting (extracting) them
with an appropriate solvent. It is usually necessary to repeat this process
a number of times in order to obtain even several milligrams of material.
Such a procedure would offer a method of identification of the various
components, however, because enough material could be collected to allow spectra
to be obtained.
Often it is useful for
the purpose of identification to run TLC chromatograms of knowns and unknowns
side by side. Other useful applications involve running multiple aliquots
of samples collected from a chromatographic column or samples taken from a
reaction mixture in order to follow reaction progress as a function of time.
Detection of spots on the
chromatogram is easy for colored materials, and a number of procedures are
available for locating spots of colorless materials. For example, irradiation
of the plate with ultraviolet light will permit location of the spots of compounds
that fluoresce. Alternatively, the solid adsorbent may be impregnated
with an otherwise inert, fluorescent substance. Spots of materials that absorb ultraviolet
light but do not fluoresce will show up as black spots against the fluorescing
background when the plate is irradiated with ultraviolet light. Other detecting agents are more often used.
These agents may be sprayed onto the chromatograms, causing the spots to become
readily apparent. Examples of detecting agents used in this way are sulfuric
acid, which causes many organic compounds to char, and potassium permanganate
solution. Iodine is another popular detecting agent. In this case the plate
is placed in a vessel whose atmosphere is saturated with iodine vapor. Iodine
is adsorbed by many organic compounds, and their spots on the chromatogram
become colored (usually brown). Since these spots usually fade, it is a good
idea to circle the spots with a pencil while they are still visible in order
to have a permanent record of the chromatogram.
Under a given set of conditions
(adsorbent, solvent, layer thickness, and homogeneity) the rate of movement
of a compound with respect to the rate of movement of the solvent front, Rf,
is a property of that compound. The value is determined by measuring the distance
traveled by a substance from a starting line in the before-mentioned figure.
The property has the same significance as retention time in a GC experiment.
A. Thin-Layer Chromatography: General Instructions
Place a TLC strip ("Bakerflex"
brand or equivalent) on a paper towel on the bench surface. Handle the strip
by the edges only and be sure the coated side is up.
Two coatings are available: Aluminum
Oxide IB-F and Silica Gel IB-F. (IB
stands for inorganic binder, and
F means that the coating contains a fluorescent indicator.)
At the top of the strip
write the letter A or S (to identify the adsorbent); also identify the solvent
to be used for development (e.g., 1:3 EA/C for 25% ethyl acetate - 75% cyclohexane).
Dip a capillary tube into
the solution of the material to be analyzed and then very carefully touch
the end of the vertically held capillary tube to the strip at a point
about 10 mm from the bottom edge. Immediately
make a mark at the side of the strip in line with the center of the spot.
Allow the solvent to evaporate completely from the spot.
Waving the strip gently in the air hastens the evaporation.
Obtain a 4-oz. round screw-cap
bottle, containing a whole or half disk of 7-cm filter paper. The cap should
be Teflon or aluminum foil lined. Pour solvent into the bottle to a depth of
no more than 5 mm. Cap the bottle
tightly and shake it vigorously to saturate the space inside with the solvent
vapor and to thoroughly wet the paper.
Carefully place the strip
(spotted end down) inside the bottle, and allow it to lean against the wall.
Close the cap tightly and let the chromatogram develop.
Do not disturb the bottle during the development.
When the solvent front
has reached a point about 1 cm from the top edge of the strip, carefully open
the bottle and remove the strip. Immediately
mark the position of the solvent front with a pencil.
Wave the strip vigorously in the air to evaporate the solvent from
the surface quickly.
Examine the strip under
ultraviolet light (in the special viewer) and outline with a pencil any spots
that are detected.
When you are finished,
do not empty the 4-oz. round screw cap bottle.
Cap the bottle tightly, see that it is properly labeled as to which
solvent it contains, and return it to the storage area.
B. Thin-Layer Chromatography: Analysis of a Commercial Analgesic
Most non-prescription pain-relieving
remedies contain aspirin (o-acetylsalicylic acid) or acetaminophen - and some
contain both. Many also have some
auxiliary compounds with analgesic properties.
In addition, caffeine is included in some preparations. Caffeine is not an analgesic, but aids in the
relief of certain types of headaches because of its stimulant effect (CNS
and cardiac).
You will receive as an
"unknown" a tablet of a commercial analgesic, to be analyzed by
TLC for the presence or absence of four specific constituents:
acetaminophen, aspirin, and caffeine. Some of the products also have other active
ingredients, but no attempt will be made to identify these other substances.
PROCEDURE
Crush the tablet to a powder
on a piece of glassine weighing paper. Put
the powder in a 5 mL plastic beaker and add 1 pipet full of methanol / dichloromethane
solution.
Stir the mixture with a
glass rod for about one minute to allow the solvent to extract the active
ingredients. Allow the insoluble matter
to settle. Carefully decant the supernatant
liquid into a clean, dry 5-mL beaker.
On a TLC strip coated with
Silica Gel IB-F, apply a spot of the solution of "unknown" and two
of the four known solutions. On a
second Silica Gel strip, spot the "unknown" solution and the remaining
two known solutions. Be sure to mark
the identity of each spot on each strip.
Develop each chromatogram
in a bottle containing 100% ethyl acetate.
Examine each strip under
the ultraviolet light viewer (short wave UV light).
Compare the spot positions (the Rf values) of the constituents of the
tablet with those of the individual authentic substances.
If very little movement of components has occurred on a strip, do not
mark any visible spots with a pencil. Replace
the strip in the bottle and repeat the development. Make a table recording the distance each substance
moved from the origin and the distance the solvent moved.
